Abstract
Background: Tab-cel is an off-the-shelf, allogeneic EBV-specific T-cell immunotherapy being investigated for EBV+ PTLD. Historical data show that in the absence of therapy, median OS is 0.7 mo in HCT recipients with EBV+ PTLD for whom rituximab (R) ± chemotherapy (CT) failed (Sanz ASH 2021) and 4.1 mo in SOT recipients with EBV+ PTLD for whom R+CT failed (Dharnidharka ASH 2021), indicating an urgent need for safe and effective therapies for this ultra-rare disease with no approved PTLD therapies. In clinical trials and expanded access programs conducted over 20 yrs in patients (pts) with relapsed/refractory (r/r) EBV+ PTLD, tab-cel has resulted in investigator-assessed ORRs of >50% and >80% 2-yr OS in pts with either CR or PR to tab-cel (Prockop JCI 2020, EBMT 2021, ATC 2021, ASH 2021). Tab-cel has been safe and well-tolerated in >180 pts with EBV+ PTLD, with tumor flare reaction (TFR) as the only identified risk (Atara Bio, Data on file). We previously presented data from ALLELE (NCT03394365) - a Phase 3, multicenter, open-label, global, registrational study - in 38 tab-cel-treated pts with EBV+ PTLD following HCT or SOT where R±CT failed. Here, we report updated outcomes in 43 pts; new data for DOR and subgroup analysis of pt baseline characteristics are also included.
Methods: In ALLELE, pts receive tab-cel at 2x106 cells/kg on days 1, 8, and 15 in 35-day cycles. Response per clinical and radiographic assessment (by PET/CT) is evaluated by independent oncologic response adjudication (IORA; primary assessment) using Lugano Classification with LYRIC modification. Efficacy endpoints include ORR, DOR, TTR, and OS. Pts are assessed up to 5 yrs for survival post treatment. These current analyses include additional pts and longer follow-up compared to previously presented ALLELE data.
Results: As of Nov 2021, 43 pts (14 HCT, 29 SOT) received at least 1 dose of study treatment and were included in the analysis. Median age was 48.5 yrs (range, 3.2-81.5). The most common PTLD subtype was diffuse large B-cell lymphoma (67.4%); 76.7% of pts had extranodal disease at screening. Among pts aged ≥16 yrs, 27.5% had an ECOG score ≥2, and 42.5%/47.5% were high/intermediate risk per PTLD-adapted prognostic index. A univariate analysis generally showed similar efficacy across various baseline characteristics; data from ongoing analyses examining ECOG (<2 vs ≥2), extranodal disease, and number of prior therapies (1 vs >1) will be presented. Median number of prior systemic treatment was 1 (range, 1-5). Pts received a median of 2 cycles (range, 1-6) of tab-cel.
Consistent with previously presented ALLELE data, overall ORR was 51.2% (22/43, 95% CI: 35.5, 66.7), 50.0% (7/14, 95% CI: 23.0, 77.0) in HCT, and 51.7% (15/29, 95% CI: 32.5, 70.6) in SOT, with a best overall response of CR (n=6, HCT; n=6, SOT) or PR (n=1, HCT; n=9, SOT; Table). Median TTR was 1.0 mo (0.7-4.7). 12/22 responders had DOR >6 mo, and median DOR was 23.0 mo (95% CI: 6.8, NE) [Table].
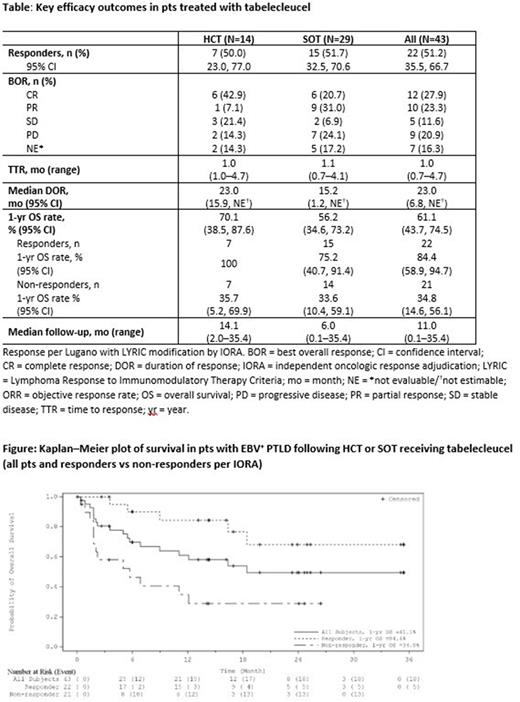
OS data were consistent with prior reports. Overall median OS was 18.4 mo (95% CI: 6.9, NE), not yet reached in HCT (95% CI: 5.7, NE), and 16.4 mo in SOT (95% CI: 5.0, NE). Overall 1-yr OS rates were 61.1% (95% CI: 43.7, 74.5) [Figure], 70.1% in HCT (95% CI: 38.5, 87.6), and 56.2% in SOT (95% CI: 34.6, 73.2) [Table]. Pts who responded had longer survival vs non-responders, with a 1-yr OS rate of 84.4% (95% CI: 58.9, 94.7) vs 34.8% (95% CI: 14.6, 56.1) and median OS of not yet reached (95% CI: 16.4, NE) vs 5.7 mo (95% CI: 1.8, NE) [Table].
Serious treatment-emergent adverse events (TEAEs) and fatal TEAEs were reported in 57.1% and 7.1% of HCT and 51.7% and 13.8% of SOT pts respectively. No fatal TEAE was treatment related. The safety profile was favorable and consistent with previous data, with no reports of TFR, infusion reactions, cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome, marrow rejection, or transmission of infectious diseases. No events of graft vs host disease or organ rejection were reported as tab-cel related.
Conclusions: Updated ALLELE Phase 3 data, which included additional pts and longer follow-up, confirm that tab-cel provides consistent clinically meaningful outcomes, including improved overall ORR and prolonged DOR and OS. Tab-cel was well-tolerated without evidence of safety concerns seen with other adoptive T-cell therapies. Tab-cel is a potentially transformative treatment advance for these pts with r/r EBV+ PTLD, for whom there are no approved therapies.
Disclosures
Mahadeo:Jazz Pharmaceuticals: Consultancy; Jazz Pharmaceuticals: Other: PI; Atara Biotherapeutics: Other: PI; Atara Biotherapeutics: Consultancy. Baiocchi:Viracta Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Atara Biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; CODIAK Biosciences: Research Funding; eLife (Journal): Other: Editorial board. Beitinjaneh:Kite/Gilead: Membership on an entity's Board of Directors or advisory committees. Chaganti:Takeda: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Pierre Fabre: Consultancy, Honoraria; Orion Pharma: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Gilead/Kite: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Atara Biotherapeutics: Consultancy, Honoraria; Adicet Bio: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Choquet:Sandoz/Novartis: Consultancy; Janssen: Consultancy; Pierre Fabre: Consultancy; Roche: Consultancy; Atara Biotherapeutics: Consultancy; Accord Healthcare: Consultancy; AbbVie: Consultancy; AstraZeneca: Consultancy; Biogaran: Consultancy; Gilead/Kite: Consultancy; Takeda: Consultancy; Viatris: Consultancy. Dierickx:Incyte: Honoraria; Roche: Research Funding; Atara Biotherapeutics: Honoraria; Novartis: Honoraria; Amgen: Honoraria; Takeda: Consultancy, Honoraria. Dinavahi:Atara Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Gamelin:Atara Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Ghobadi:Amgen: Consultancy, Research Funding; Atara: Consultancy; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Wugen Inc: Consultancy; Celgene: Consultancy; CRISPR Therapeutics: Consultancy; Genentech: Research Funding. Guzman-Becerra:Atara Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Joshi:Atara Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Mehta:Atara Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Nikiforow:GlaxoSmithKline: Consultancy; Iovance: Consultancy; Kite/Gilead: Consultancy. Reshef:Celgene: Research Funding; Pfizer: Consultancy; Magneta: Consultancy; Kite: Consultancy, Research Funding; Bayer: Consultancy; TScan: Consultancy; Synthekine: Consultancy; Regeneron: Consultancy; Novartis: Consultancy; Jasper: Consultancy; Bristol-Myers Squibb: Consultancy; Takeda: Research Funding; Shire: Research Funding; Precision Biosciences: Research Funding; Pharmacyclics: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Immatics: Research Funding; J&J: Research Funding; Gilead Science: Consultancy, Research Funding; Atara Biotherapeutics: Consultancy, Research Funding. Ye:Atara Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Prockop:Memorial Sloan Kettering Cancer Center (MSK): Other: Co-inventor of intellectual property transferred from Atara; AlloVir (through MSK): Research Funding; Jasper Therapeutics (through MSK): Research Funding; Atara Biotherapeutics (through MSK): Other: Co-inventor of IP licensed to Atara. Dr Prockop transferred IP rights to MSK & has no personal financial interests in Atara. MSK has financial interests in Atara/IP interests related to this study, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal